- All Atoms Of The Same Element Have The Same Atomic Mass
- All Atoms Of The Same Element Have The Same Number Of Protons
- All Atoms Of The Same Element Have The Same Number Of
- All Atoms Of The Same Element Have The Same Mass Number
- All Atoms Of The Same Element Have The Same Number Of Protons
- All Atoms Of The Same Element Have The Same Number Of Positive Subatomic Particles
Each and every element has a unique number known as atomic number and atomic number is number is identified by the number of protons that are present in an atom. All atoms of the same element has same number of protons. For Example: All atoms of sodium will have 11 number of protons. Hence, the correct answer is protons. When you have finished the test, click on the ANSWERS button and see how well you have done. Test Number PS-1030. Quick Questions. 1) All atoms are the same. 2) All atoms of the same element are the same. 3) All atoms contain neutrons. 4) Protons have more mass than neutrons.


- All atoms of the same element have the same. Number of neutrons C. Atomic number 1 See answer yep it didn't work. Geologic and fossil evidence suggests that all landmasses were once connected in a supercontinent named.
- The same number of protons. Atoms of same element must have same number of protons, same number of electrons but may also have same or different number of neutrons (in case of isotopes). Answer verified by Toppr.
- Correct answers: 2 question: All atoms of the same element must have the same number of A. I love you, thxxx ⭐️.
Chemistry please check
1. Which of the following would occur as saturated hydrocarbons? alkanes alkenes alkynes cycloalkynes ans: alkanes 2. There are millions of organic compounds but only thousands of inorganic compounds because atoms of elements
chemistry
Which statement best describes what must occur for two atoms to combine to form a chemical bond? A. The electrons of one nucleus must transfer to the nucleus of the second atom for a chemical bond to form. B. The repulsive forces
SCIENCE --- CHECK MY WORK PLS
help pls, check my work asap!?? 1) Which of the following is a chemical change? (1 point) Boiling of water*** Melting a cube of ice Dissolving 10 grams of salt in water Splitting water into hydrogen and oxygen 2) What is the mass
Chemistry
Write the chemical formulas for the compounds containing each of the following: a) one calcium atom for every two iodide atoms. b) two nitrogen atoms for every four oxygen atoms. c) one silicon atom for every two oxygen atoms. d)
You can view more similar questions or ask a new question.
All Atoms Of The Same Element Have The Same Atomic Mass
Science at a Distance| Lecture Notes - Part 1 | Physical Structure |
Quick Check
Read each of the Quick Questions below and write down your answer.
When you have finished the test, click on the ANSWERS button and see how well you have done.
Test Number PS-1030
Quick Questions
1) All atoms are the same. (True / False)?
2) All atoms of the same element are the same. (True / False)?
3) All atoms contain neutrons. (True / False)?
4) Protons have more mass than neutrons. (True / False)?
5) Electrons have less mass than either protons or neutrons. (True / False)?
6) In a neutral atom there are always equal numbers of protons and electrons. (True / False)?
7) Isotopes are a family of atoms all of which have the same number of electrons. (True / False)? How to change macbook keyboard language.
8) Electrons are found in clouds called orbitals surrounding the atomic centers. (True / False)?
9) Electrons are sometimes exchanged between atoms. (True / False)? Cdj 800 rekordbox.
10) Ions are atoms that have gained or lost electrons. (True / False)?
11) Ions always have a positive charge. (True / False)?
12) Atoms are at their most stable when their outermost electron energy level is completely filled or completely empty of electrons. (True / False)?
13) Hydrogen atoms and Helium atoms both have two protons in their atomic centers, but different numbers of neutrons. (True / False)?
14) Hydrogen and Deuterium atoms both have the same number of protons in their atomic centers, but different numbers of neutrons. (True / False)?
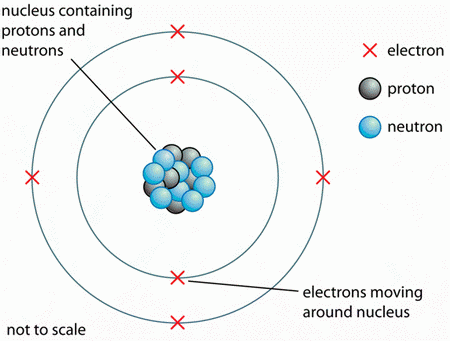
15) The atomic mass of all isotopes within a family of atoms is always the same. (True / False)?
16) The atomic number of all isotopes within a family of atoms is always the same. (True / False)?
17) Atoms of the same element can sometimes share two electrons. (True / False)?
18) Atoms of different elements can sometimes share electrons. (True / False)?
19) Atoms share electrons so as to fill energy levels. (True / False)?
20) Only eight electrons can occupy an one orbital. (True / False)?
21) Two electrons shared between two atoms creates a force or bond that holds the atoms together. (True / False)?
All Atoms Of The Same Element Have The Same Number Of Protons
22) More than two electrons are sometimes shared between atoms. (True / False)?
23) Covalent bonds can only form between atoms that can ionize. (True / False)?
24) Ionization of a hydrogen atom produces a proton. (True / False)?
25) Methane is an isotope of carbon. (True / False)?
All Atoms Of The Same Element Have The Same Number Of
26) Water molecules are smaller than molecules of oxygen gas. (True / False)?
27) Hydrogen and oxygen share the electrons equally in the covalent bonds of a water molecule. (True / False)?
28) A polar molecule is one that is most stable at the poles of reaction. (True / False)?
All Atoms Of The Same Element Have The Same Mass Number
29) Water is a liquid at room temperature because the water molecules are strongly sympathetic to one another. (True / False)?
30) Hydrophilic substances dissolve readily and easily in water. (True / False)?
All Atoms Of The Same Element Have The Same Number Of Protons
| Answers to Part 1 | Lecture Notes - Part 1 |
| Physical Structure | |
All Atoms Of The Same Element Have The Same Number Of Positive Subatomic Particles
Science at a Distance© 1997, 1998, 1999, 2000, Professor John Blamire
